LL-37 – Research Overview
LL-37 is a naturally occurring cationic antimicrobial peptide (AMP) derived from the human cathelicidin precursor protein hCAP-18. It has been extensively studied in preclinical and laboratory research for its role in innate immune defense, barrier integrity signaling, and inflammatory pathway modulation. Due to its broad biological relevance, LL-37 is frequently referenced in immunology research, host defense studies, wound-healing investigations, and epithelial barrier signaling research.
This page provides a research-focused, educational overview of LL-37, including its molecular classification, mechanism of action in research contexts, and primary areas of scientific investigation.
⚠️ Research Disclaimer:
This content is provided strictly for educational and research purposes. No information on this page constitutes medical advice, dosing guidance, or instructions for human or animal use.
Compound Overview
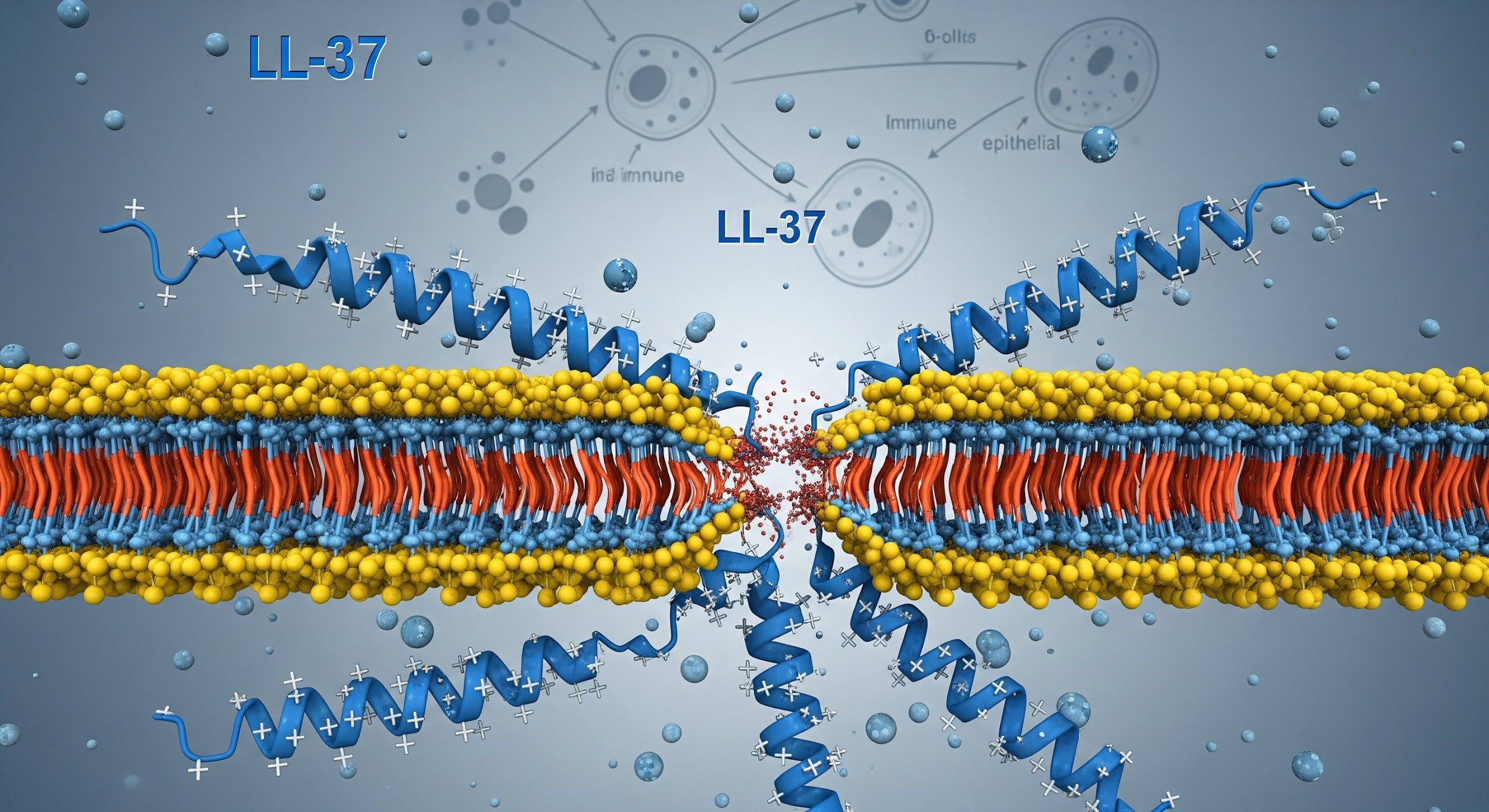
LL-37 is classified as a 37-amino-acid antimicrobial peptide and represents the only known human cathelicidin. In laboratory research environments, LL-37 is studied for its role in innate immune signaling, microbial defense mechanisms, and immune-epithelial communication pathways. Beyond direct antimicrobial activity, LL-37 is of significant interest due to its involvement in immune modulation, chemotactic signaling, and tissue repair-associated pathways, making it a multifunctional peptide in immune research.Research Background & Classification
From a molecular research perspective, LL-37 belongs to the antimicrobial peptide (AMP) family, a critical component of the innate immune system. Researchers study LL-37 to explore how AMPs contribute to:- First-line immune defense mechanisms
- Host–pathogen interaction signaling
- Immune cell recruitment and activation
- Epithelial barrier protection
- Inflammatory response regulation
Mechanism of Action (Research Context)
In laboratory research settings, LL-37 has been studied for its ability to interact with microbial membranes, contributing to antimicrobial defense, while also influencing immune signaling pathways. Researchers analyze how LL-37 affects:- Chemotaxis of immune cells
- Cytokine and chemokine signaling
- Toll-like receptor (TLR) pathway modulation
- Epithelial cell signaling and barrier repair
- Inflammatory signaling balance
Areas of Scientific Research Interest
LL-37 has been referenced in scientific research related to:
- Innate immune defense signaling
- Antimicrobial peptide (AMP) research
- Host–pathogen interaction studies
- Epithelial barrier integrity signaling
- Wound-healing pathway investigations
- Inflammatory and immune modulation research
- Chemotactic signaling and immune cell recruitment
- Skin, gut, and mucosal immunity studies
These areas support broader investigation into how antimicrobial peptides regulate immune defense and tissue protection mechanisms.
Stability & Handling Considerations
In laboratory environments, LL-37 is handled according to standard peptide research protocols. Researchers consider factors such as temperature stability, light exposure, solution composition, and peptide aggregation risk when designing experiments.
Proper handling and storage are essential to preserve molecular integrity and signaling consistency during immune-focused research studies.
Research Context Notes
This overview is intended for educational and informational purposes for individuals studying immunology, molecular biology, host defense mechanisms, and barrier immunity pathways. It does not replace peer-reviewed scientific literature, experimental protocols, regulatory documentation, or institutional research standards.


